News
Promising results announced from GSK’s shingles trial in China
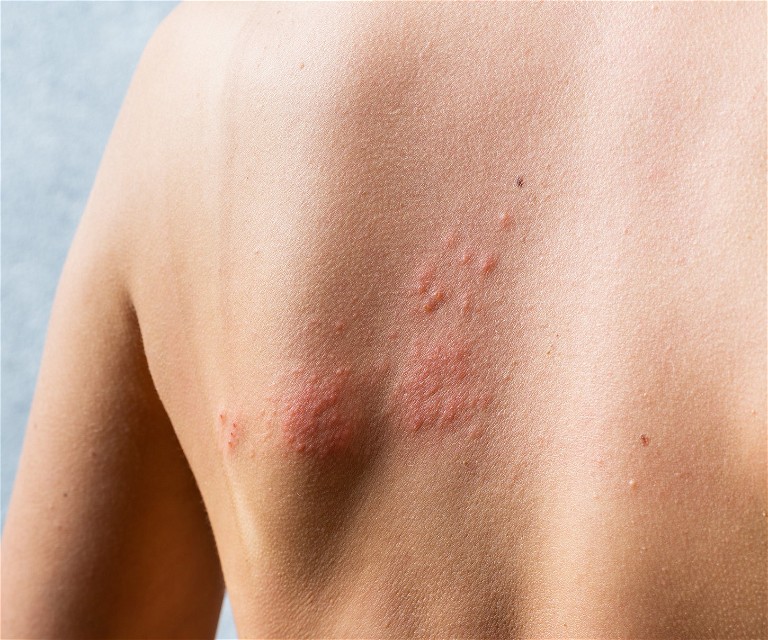
GSK has announced positive results from ZOSTER-076 post-licence phase 4 efficacy trial for Shingrix recombinant zoster vaccine (RZV) in China. The trial evaluated the vaccine’s efficacy at preventing shingles in 6,000 participants over the age of 50; the participants who received the Shingrix vaccination reported no cases, whereas those who were given placebo reported 31 cases.
GSK also carried out the phase 3 trials ZOE-50 and ZOE-70, which demonstrated how the RZV vaccine’s efficacy was up to 97% over the length of four years. RZV was originally used in 2019 for the prevention of herpes zoster in patients over 50, and trials were consequently put in place for the treatment of shingles after its success – the current trials further demonstrate the vaccine’s efficacy for preventing herpes, regardless of sex, ethnicity or geographical region.
The varicella zoster virus (VZV) that causes shingles and chicken pox is typically present in over 90% of adults globally; it remains in the nervous system until it attempts to reactivate as shingles as age increases. As the population in China increases, the six million cases of shingles annually are also expected to increase, making this a rising issue for people over the age of 65.
The results from the phase 4 trial are expected to be published in a peer-reviewed scientific journal later in 2023.