46 articles from this collection:
Measles outbreak possible, advises UKHSA
The UKHSA has warned that further outbreaks of measles could spread to other towns and cities unless uptake in the measles, mumps and rubella vaccination is increased in at-risk areas.

PharmaTimes
Www.pharmatimes.com/intcr
FDA approves skin cancer AI detection device
DermaSensor has announced that the FDA has granted clearance for its real-time, non-invasive skin cancer evaluation system.

SAMEDAN - ICT
International Clinical Trials

Contents
Welcome to the January/February 2024 issue of Pharmafocus!

Comment
Welcome to the January/February 2024 issue of Pharmafocus!

Pharma Role
Pharmarole.com

BioNTech’s Rwanda vaccine manufacturing site achieves milestone
BioNTech has announced that it has reached the next milestone in the establishment of its mRNA vaccine manufacturing capacities in Africa, with the introduction of the new site in Kigali, Rwanda.
Digital healthcare platform launched by Eli Lilly
Eli Lilly has announced its new digital healthcare experience, LillyDirect, which will benefit patients in the US with obesity, migraine and diabetes. This is intended to offer resources around disease management, such as access to healthcare providers, tailored support and delivery of selected medications.

AstraZeneca pays C4X an $11m milestone payment
C4X Discovery Holdings has announced that it has received a milestone payment of $11m from AstraZeneca, which was triggered by the preclinical progress of C4XD’s NRF2 activator programme.
FDA grants Fast Track Designation for Verastem’s combination NSCLC treatment
Verastem Oncology has announced that the FDA has granted fast track designation for the company’s investigational raf/mek clamp, avutometinib, in combination with amgen’s kras g12c inhibitor, lumakras (sotorasib) for the treatment of patients with kras g12c-mutant metastatic non-small cell lung cancer.
GSK’s Jemperli plus Zejula combination performs well in trial as endometrial cancer treatment
GSK has announced positive results from a planned analysis of the second part of the RUBY/ENGOT-EN6/GOG3031/NSGO phase 3 trial

Novo Nordisk announces data from COMBINE 3 phase 3a trial
Novo Nordisk has shared topline results from the COMBINE 3 phase 3a trial of once-weekly Icosema, a fixed-ratio combination treatment consisting of basal insulin icodec and semaglutide.

Daiichi Sankyo shares data from clinical trial for Ezharmia as lymphoma treatment
Daiichi Sankyo has announced results from the phase 2 valentine-ptcl01 trial of Ezharmia (valemetostat tosilate), which demonstrated a clinically meaningful and durable response in patients with relapsed or refractory peripheral t-cell lymphoma.

Regeneron shares results from trial for multiple myeloma treatment
Regeneron Pharmaceuticals has announced positive data from its pivotal linker-mm1 trial, which evaluated linvoseltamab as a treatment for adult patients with relapsed/refractory multiple myeloma.

FLYPHARMA EUROPE
Www.flypharmaeurope.com
Merck’s Keytruda with chemoradiotherapy approved by FDA as treatment for cervical cancer
Merck has announced that the FDA has approved Keytruda (pembrolizumab) in combination with chemoradiotherapy for the treatment of figo (international federation of gynecology and obstetrics) 2014 stage iii-iva cervical cancer.

J&J’s Balversa approved by FDA for bladder cancer treatment
Johnson & Johnson (J&J) has announced that the FDA has approved a supplemental new drug application for Balversa (erdafitinib) for the treatment of adult patients with locally advanced or metastatic urothelial carcinoma with susceptible fibroblast growth factor receptor 3 genetic alterations whose disease has progressed on or after one prior treatment.
Pharmafile
Www.pharmafile.com

AstraZeneca and Ionis’s Wainua approved by FDA
AstraZeneca and Ionis have announced that the FDA has approved Wainua (eplontersen) for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis (haattr-pn or attrv-pn) in adult patients.

FDA approves Calliditas Therapeutics’ Tarpeyo to reduce loss of kidney function
Calliditas Therapeutics has announced that the FDA has approved Tarpeyo (budesonide) delayed release capsules to reduce the loss of kidney function in adults with primary immunoglobin a nephropathy at risk of disease progression.

COMMUNIQUÉ
Communique.awardsplatform.com

Gilead and Compugen announce new agreement for immunotherapy programme
Gilead Sciences and Compugen have announced that they have entered an exclusive licence agreement for Compugen’s potential first in class, preclinical antibody programme against il-18 binding protein, including the com503 drug candidate.

Genetech and NVIDIA announce collaboration using AI for drug discovery and development
Genentech has announced that it has entered into a multi-year strategic research collaboration with NVIDIA that is intended to pair Genentech’s artificial intelligence (AI) capabilities, biological and molecular data sets and research expertise with NVIDIA's accelerated computing capabilities and AI in order to speed up drug discovery and development.

SAMEDAN
Www.samedanltd.com

Owkin and Merck collaborate on cancer diagnostics powered by AI
Owkin and Merck, known as MSD outside of the US and Canada, have announced that they have entered into a collaboration agreement for the development and commercialisation of AI-powered digital pathology diagnostics for the European Union (EU) market.

Alphamab Oncology and 3DMedicines announce licensing agreement with Glenmark Speciality for antibody
Alphamab Oncology and 3DMedicines have announced that they have entered into a licence agreement with Glenmark Speciality, a subsidiary of Glenmark Pharmaceuticals, for the subcutaneous injection pd-l1 antibody drug, known as envafolimab.

Inhibrx to be acquired by Sanofi for approximately $1.7bn
Sanofi and Inhibrx, Inc have announced that they have entered into a definitive agreement for Sanofi to acquire Inhibrx following the spin-off of non-inbrx-101 assets into New Inhibrx.

Aiolos Bio to be acquired by GSK for $1bn
GSK and Aiolos Bio have announced that they have entered into an agreement for GSK to acquire Aiolos for an initial $1bn upfront payment along with up to $400m in success-based regulatory milestone payments.

Harpoon Therapeutics set to be acquired by Merck for approximately $680m
Merck (known as MSD outside of the US and Canada) and Harpoon Therapeutics have announced that they have entered a definitive agreement for Merck, through a subsidiary, to acquire Harpoon for an approximate total equity value of $680m or $23 per share.

The risk of replicating social bias in synthetic patients – the need for human intelligence with AI
Paul Reed and Annabelle Gall at Research Partnership, an Inizio Advisory company, consider the benefits and drawbacks of AI, especially in terms of diversity and synthetic patients
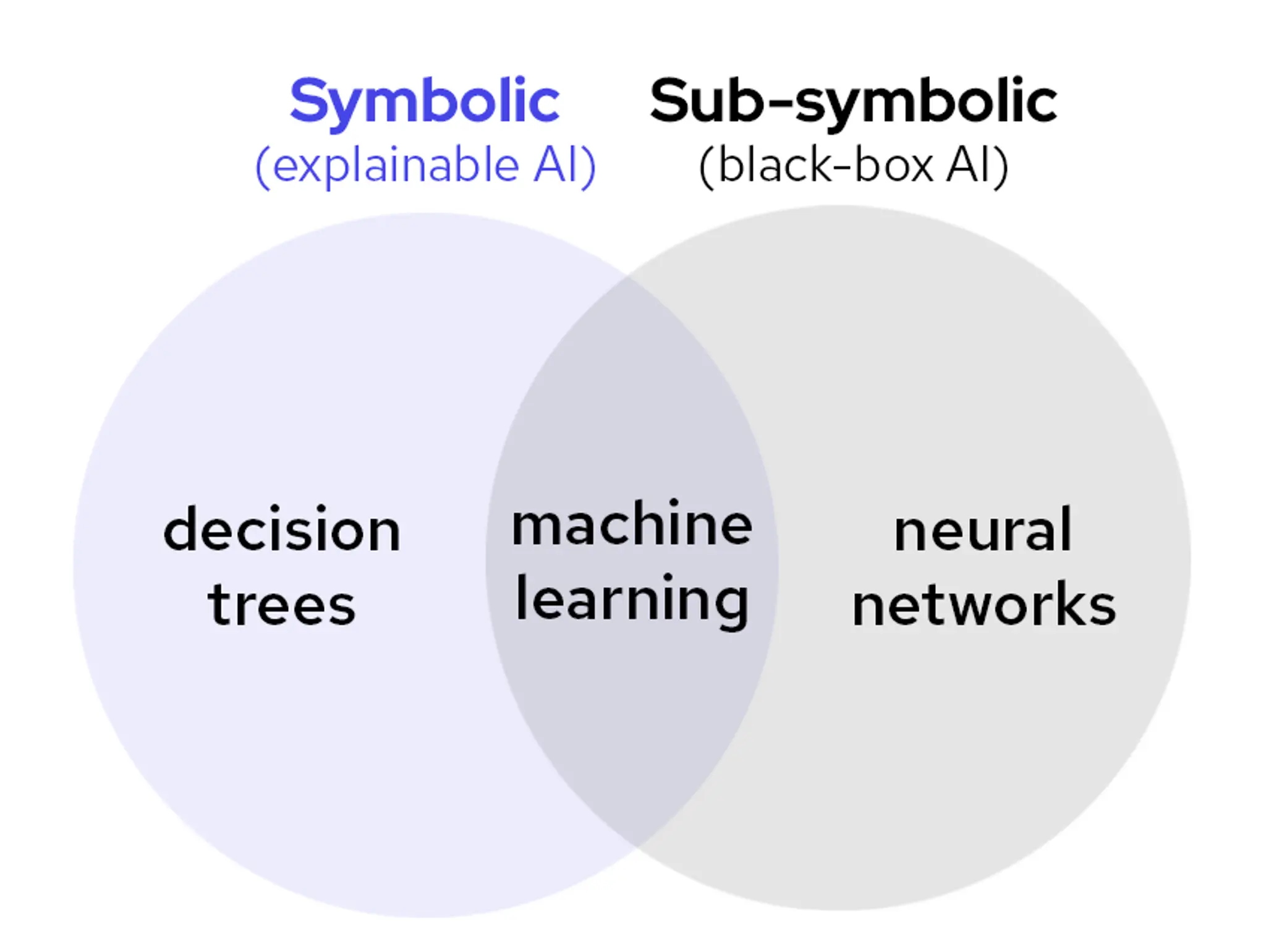
Don’t settle for black box: why only explainable AI is built for scientific discovery
Lykke Pedersen from Abzu considers the benefits of AI and its different applications within the pharma industry

2024: a tipping point for progress in rare diseases?
Soraya Bekkali from Alexion, AstraZeneca Rare Disease, explains why the EU must act in 2024 to tackle the health equity crisis that people living with rare diseases face every day

Pharmatimes
Www.pharmatimes.com/croy

Paediatrics in the review of the EU General Pharmaceutical Legislation
Pauline Roudot from Novartis considers the path to approval for paediatric medications
The impact of climate change on respiratory health
With the first ever COP health event being held in December 2023, Shishir Patel from Chiesi UK and Ireland reflects on the need to align environmental and health strategies and shares his work building a clearer picture of how climate change can impact respiratory health

Ageing populations and geographical health disparities in the UK
Betsy Goodfellow from Pharmafocus considers how the UK’s ageing population impacts general health trends, focusing on how a greater proportion of people’s lives are spent in ill health as more people are living much longer

Our Winter of Discontent: NHS strikes and missed targets
Betsy Goodfellow from Pharmafocus considers the current state of the NHS amid ongoing strikes and ever-growing waiting lists, as well as the impact of the infectious illnesses circulating during the winter months

Samedan Ltd
Www.samedanltd.com

Dr Joerg Moeller appointed CEO of BenevolentAI
BenevolentAI has announced that it has appointed Dr Joerg Moeller MD PhD as its new chief executive officer and executive board member, the changes will be effective immediately.

Sosei Group appoints Toshihiro Maeda as COO
Sosei Group corporation has announced the appointment of Toshihiro Maeda as chief operating officer (COO).

X-Chem announces new CEO and CSO
X-Chem has announced that it is making changes to its executive management team, with Karen Lackey, the current chief scientific officer (CSO), becoming chief executive officer (CEO) and Matthew Clark, the current CEO pivoting to the role of president and CSO.

Peter Crossley appointed COO of Cellular Origins
Cellular Origins has announced that it has appointed Peter Crossley as chief operating officer (COO).

Five facts about the NHS
Five key facts about the NHS
Pharmafocus - X.com
Get in touch @pharmafocus

Pharmafile
Www.pharmafile.com
