45 articles from this collection:
FDA approves AstraZeneca’s Tagrisso for lung cancer treatment
AstraZeneca has announced that the FDA has approved Tagrisso for the treatment of adult patients with locally advanced or metastatic epidermal growth factor receptor-mutated non-small cell lung cancer.

PharmaTimes
Www.pharmatimes.com/intcr
$254m funding raised by Freenome for early cancer detection
Freenome has announced that it has raised $254m from new and existing investors, intended to be used for the advancement of its single-cancer and tailored multi-cancer early detection tests developed on its multiomics platform.

International Clinical Trials
Samedan ltd presents International Clinical Trials (ICT)

Contents
Welcome to the March 2024 issue of Pharmafocus!

Comment
Considering the increase in measles cases and the benefits of vaccination

PharmaRole
Pharmarole.com
FDA grants Fast Track designation to GSK’s chronic hepatitis B treatment
GSK has announced that the FDA has granted fast track designation for bepirovirsen, an investigational antisense oligonucleotide for the treatment of chronic hepatitis b.
FDA grants breakthrough therapy designation for J&J’s nipocalimab as HDFN treatment
Johnson & Johnson has announced that the FDA has granted breakthrough therapy designation for nipocalimab for the treatment of alloimmunised pregnant individuals at high risk of severe haemolytic disease of the foetus and newborn.
FDA accepts argenx’s sBLA for Vyvgart Hytrulo for CIDP treatment
Argenx has announced that the FDA has accepted a supplemental biologics license application (sBLA) for Vyvgart Hytrulo (efgartigimod alfa and hyaluronidase-qvfc) for priority review.

Domainex announces opening of new Biology Centre of Excellence in Cambridge, UK
Domainex has announced that it has opened a new biology centre of excellence at the Unity Campus at Pampisford, Cambridge, UK, in order to further support collaborations with pharmaceutical and biotechnology companies, patient foundations and academic institutions.

Roche announces results from phase 3 study of allergy treatment, Xolair
Roche has announced data from stage 1 of the NIH-sponsored phase 3 outmatch trial, which assessed the safety and efficacy of Xolair (omalizumab) in patients allergic to peanuts and at least two other common foods.
Boehringer Ingelheim shares data from liver disease phase 2 trial
Boehringer Ingelheim has announced results from the phase 2 trial of Survodutide for the treatment of liver disease due to metabolic dysfunction-associated steatohepatitis (MASH).

ViiV Healthcare announces interim results from phase 3 trial for HIV treatment
ViiV Healthcare has announced results from an interim analysis of the phase 3 LATITUDE trial, which assessed Cabenuva (cabotegravirandrilpivirine), a long-acting injectable antiretroviral treatment (ART) for HIV treatment.

First patient enrolled in Tenax Therapeutics’ phase 3 trial for pulmonary hypertension treatment
Tenax Therapeutics has announced that it has enrolled the first patient in its phase 3 level study, which aims to assess TNX-103 (oral levosimendan) for the treatment of pulmonary hypertension in heart failure with preserved ejection fraction.

FLYPHARMA
Www.flypharmaeurope.com
European Commission approves Pfizer’s Velsipity for ulcerative colitis treatment
Pfizer has announced that the EC has granted marketing authorisation for Velsipity (etrasimod) in the EU.

Ipsen’s Onivyde approved by FDA for treatment of pancreatic adenocarcinoma
Ipsen has announced that the FDA has approved the supplemental new drug application for Onivyde (irinotecan liposome injection) plus oxaliplatin, fluorouracil and ;eucovorin (Nalirifox) as a first-line treatment for adult patients with metastatic pancreatic adenocarcinoma (mpdac).
Pharmafile
Www.pharmafile.com

AbbVie’s Tepkinly recommended by NICE for SC treatment of DLBCL
AbbVie has announced that the NICE has recommended Tepkinly (epcoritamab) as a treatment option for adults with diffuse large b-cell lymphoma (DLBCL) whose cancer has returned or not responded to two previous treatments.

Vertex’s Casgevy for sickle cell disease treatment approved by European Commission
Vertex Pharmaceuticals has announced that the European Commission (EC) has granted conditional marketing authorisation to Casgevy (exagamglogene autotemcel), a crispr/cas9 gene-edited therapy.

COMMUNIQUE
Communique.awardsplatform.com
AbbVie and Tentarix announce oncology and immunology collaboration
AbbVie and Tentarix Biotherapeutics have announced that they have entered a multi-year collaboration focused on the discovery and development of conditionally active, multi-specific biologic candidates in oncology and immunology.

Astellas and Kelonia Therapeutics announce agreement for immunooncology therapeutics
Astellas Pharma and Kelonia Therapeutics have announced that Xyphos Biosciences (a wholly owned subsidiary of Astellas) and Kelonia have entered into a research collaboration and licence agreement for the development of novel immuno-oncology therapeutics.

SAMEDAN
Www.samedanltd.com
BioNTech and Autolus collaborate for CAR T-cell therapies
BioNTech and Autolus Therapeutics have announced that they have entered a strategic collaboration intended to further both companies’ autologous CAR T programmes towards commercialisation following regulatory authorisations.
€4.9m raised for development of Vivet Therapeutics’ gene therapies
Vivet Therapeutics has announced that it has received €4.9m financing from the French Government for the advanced development of a gene therapy for the treatment of cerebrotendinous xanthomatosis (CTX), a rare neurodegenerative disease.

MorphoSys to be acquired by Novartis for €2.7bn
Novartis has announced that it has entered an agreement to acquire Morphosys AG for €2.7bn, or €68 per share.
Eli Lilly’s Cialis and Alimta to be acquired by Zuellig Pharma in certain ASEAN markets
Zuellig Pharma has announced that it has completed the acquisition of two brands, Cialis (tadalafil) and Alita (pemetrexed), from Eli Lilly for selected ASEAN markets, including Brunei, Cambodia, Indonesia, Laos, Malaysia, Myanmar, Philippines and Thailand.

Jazz Pharmaceuticals to acquire rights to Redx Pharma’s KRAS inhibitor programme
Jazz Pharmaceuticals and Redx Pharma have announced that the companies have signed a definitive agreement under which Jazz will acquire Redx’s kirsten rat sarcoma virus (KRAS) inhibitor programme.

PharmaTimes
Www.pharmatimes.com/croy
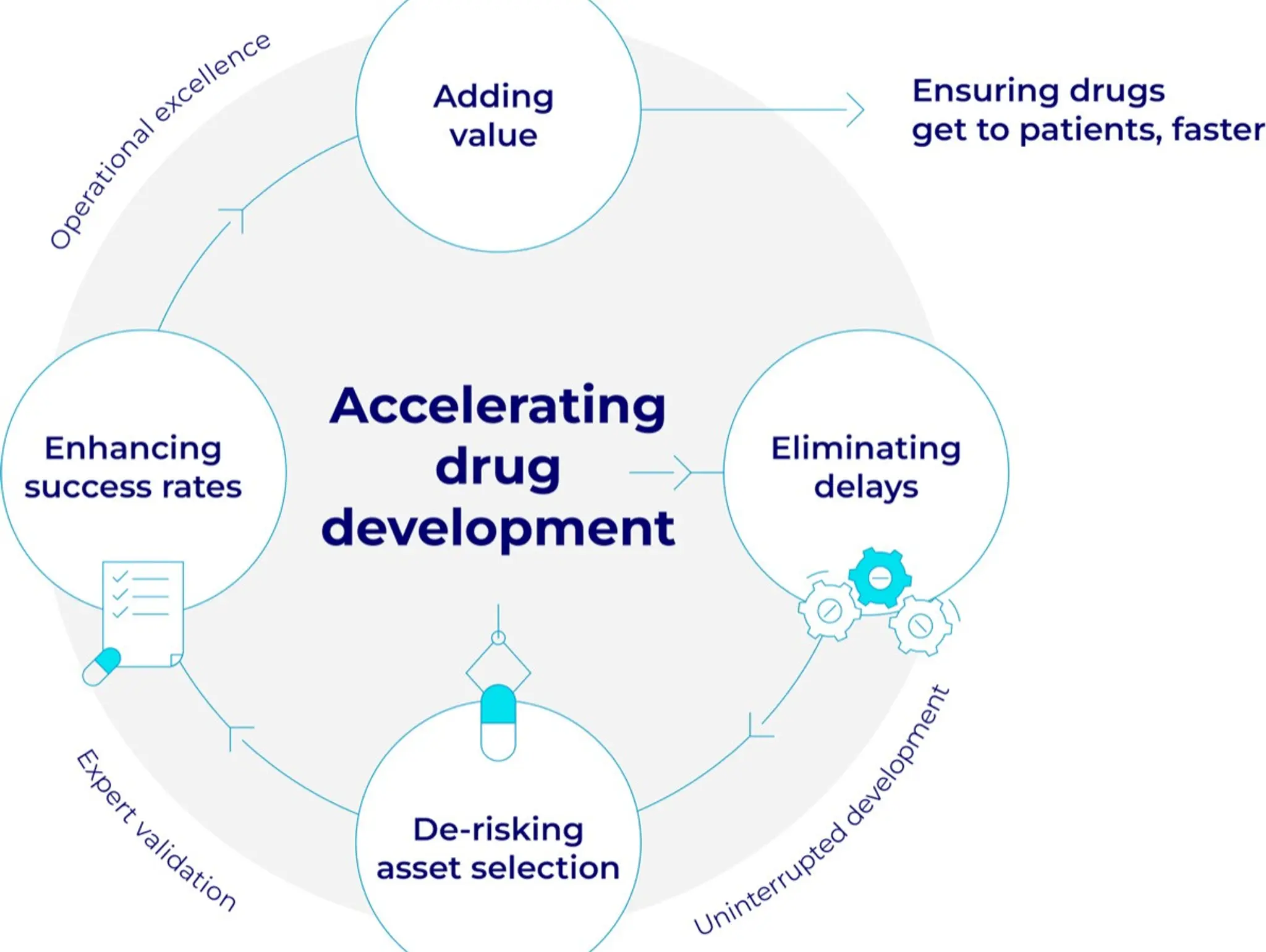
Next generation treatments and clinical trials for non-small cell lung cancer
Professor Tobias Arkenau from Ellipses Pharma explores the need and potential for next generation treatments as well as the global need to keep improving the clinical trial structure and landscape
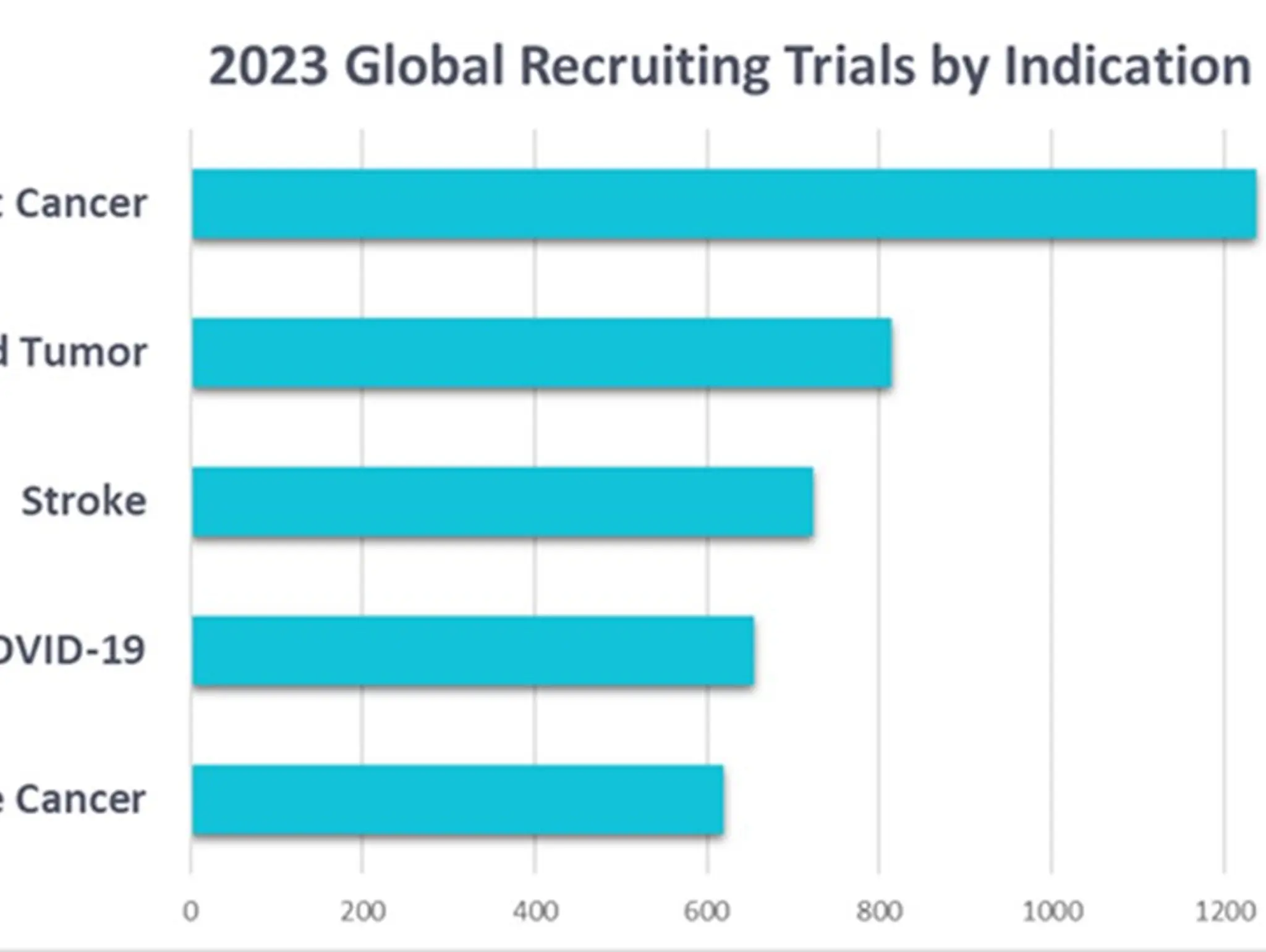
Bridging science and data: a new era in cancer clinical trials
Dr Gen Li, president at Phesi, considers patient enrolment and retention in clinical trials, with a focus on oncology trials and the challenges facing them

The landscape of blood cancer treatments in the UK
Pharmafocus speaks to Gilead Sciences about the current treatments available for blood cancers and how they are likely to develop in the future

Community pharmacies need more support to deliver Pharmacy First scheme
Santosh Sahu at Charac considers the NHS’ Pharmacy First scheme, it benefits and the barriers to its implementation

Streamlining patient support programmes: a five-step approach
Emma Bishop at Cognitant Group explores the need for patient support programmes, and how these can be improved
Measles: the UK’s next epidemic?
Betsy Goodfellow from Pharmafocus considers the current rise of measles cases in the UK and the measures that should be taken to prevent further infections

SAMEDAN
Www.samedanltd.com

Amylyx Pharmaceuticals appoints Andrew Caldwell as General Manager UK and Ireland
Amylyx Pharmaceuticals has announced that it has appointed Andrew Caldwell to general manager UK and Ireland.

Georges Rawadi appointed CEO of StromaCare
French biotech company StromaCare has announced the appointment of Georges Rawadi as chief executive officer (CEO).

Cuttsy+Cuttsy announces appointment of Jon Hume as commercial director
Cuttsy+Cuttsy has announced the appointment of Jon Hume as commercial director, taking his new role from 1 March 2024.

AdhereTech appoints Paul Sekhri to board of directors
AdhereTech has announced that it has appointed Paul Sekhri to its board of directors, having begun his new role on 7 February 2024.
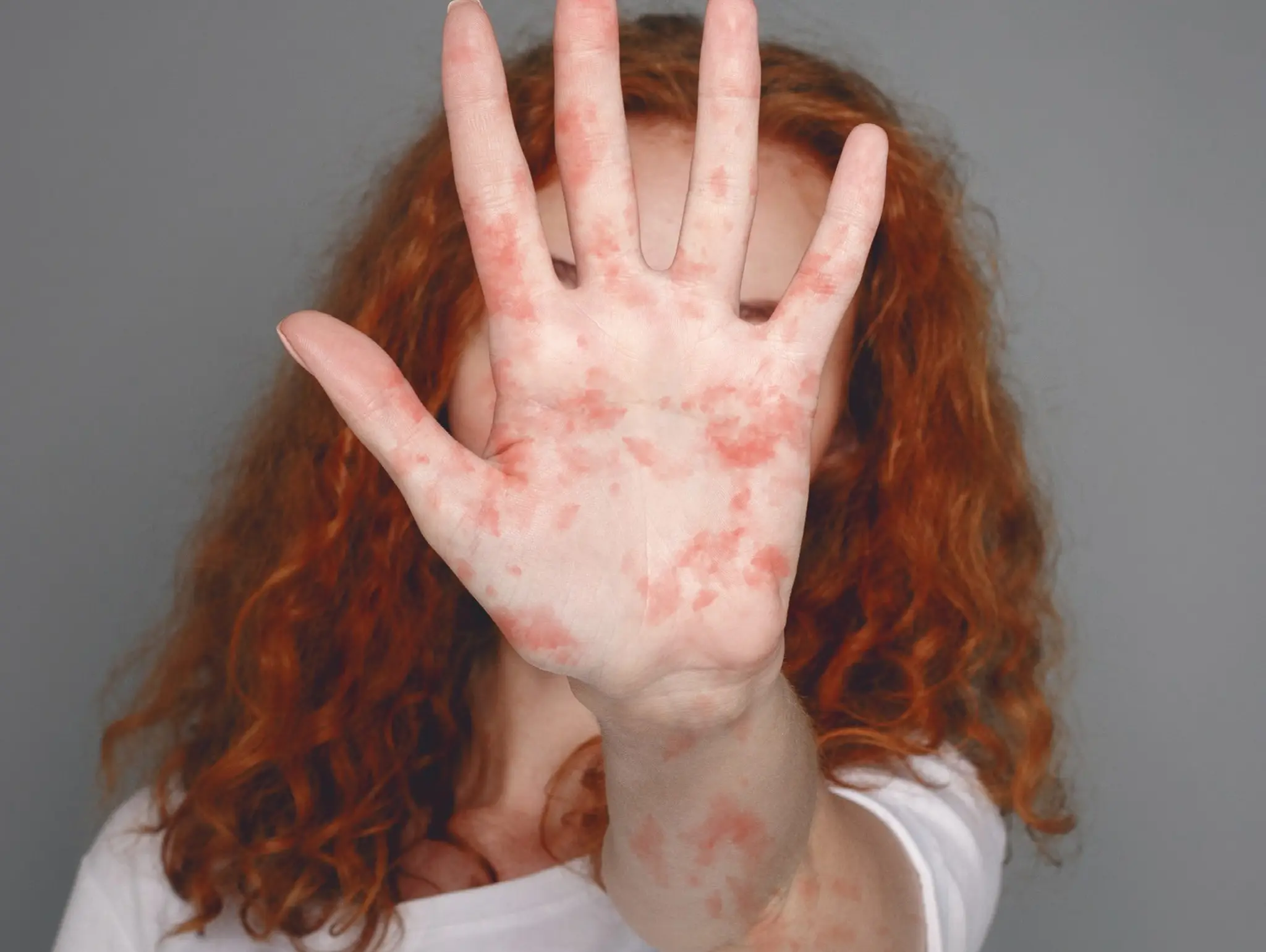
Five facts about measles
Five key facts about measles
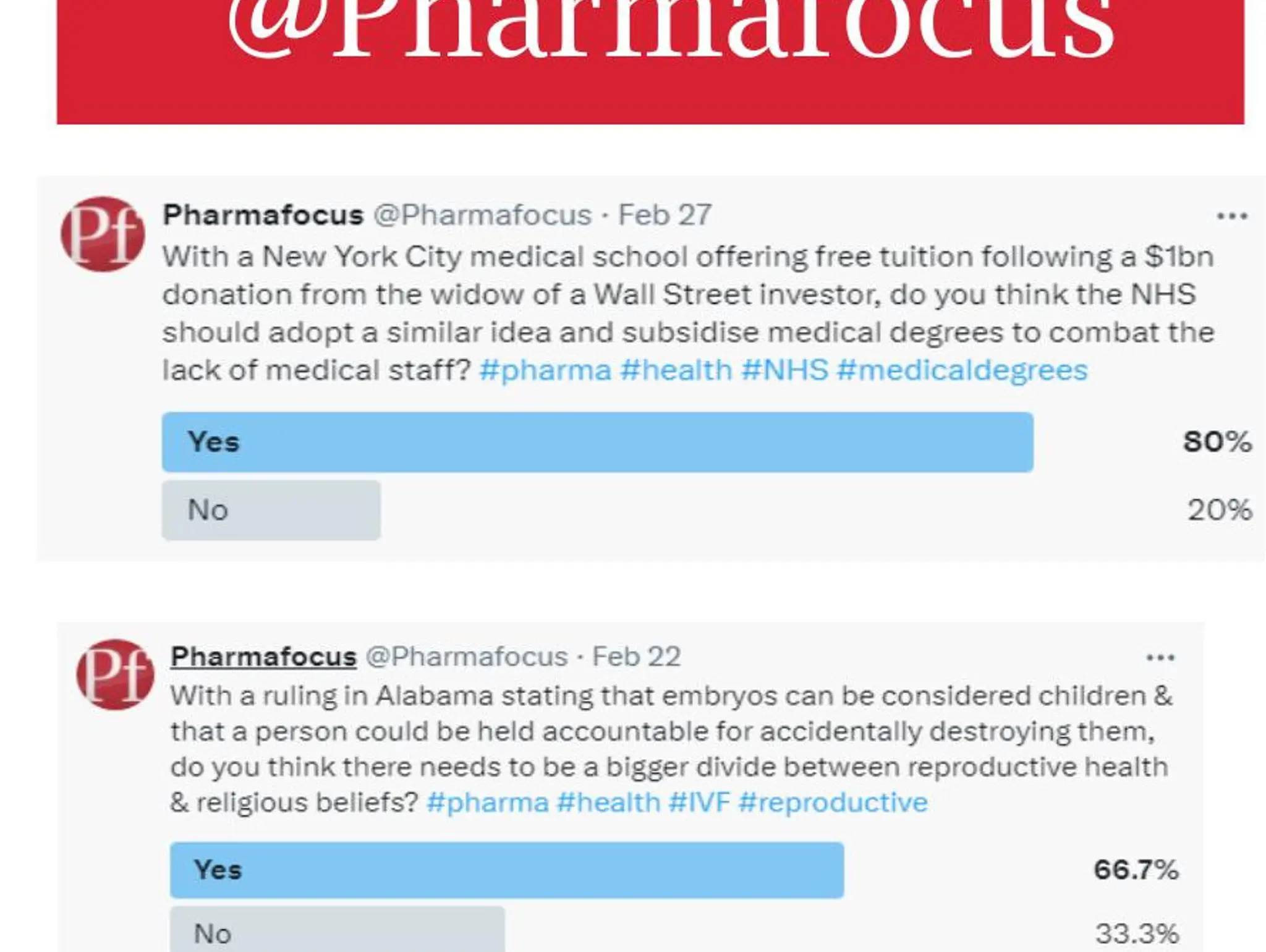
Get in touch @Pharmafocus
Get in touch @Pharmafocus on x.com

Pharmafile
Www.pharmafile.com
