45 articles from this collection:

Janssen’s Talvey approved by FDA for the treatment of multiple myeloma
The Janssen Pharmaceutical Companies of Johnson & Johnson has announced that the FDA has granted accelerated approval of Talvey (talquetamab-tgvs) for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four previous therapies.

PMEA
Https://pmea.awardsplatform.com/
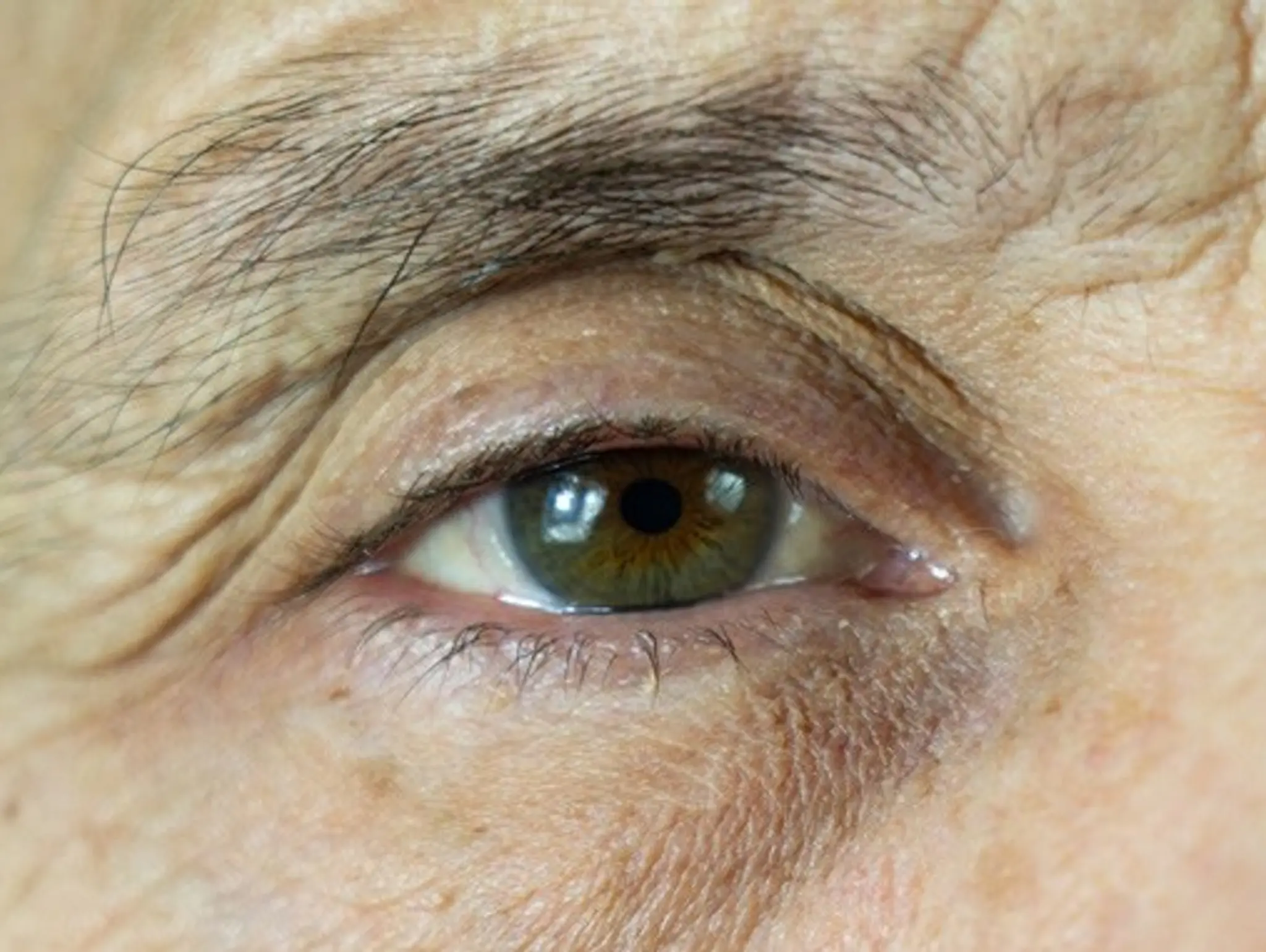
Regeneron announces two-year results from PULSAR trial for wet age-related macular degeneration
Regeneron Pharmaceuticals has announced positive, two-year, topline results from its pulsar trial assessing aflibercept 8mg for the treatment of patients with wet age-related macular degeneration (WAMD).

Pharmatimes
Pharmatimes.com/communications_awards

Contents
October Pharmafocus includes the latest news as well as fascinating articles.

Comment
Welcome to the October issue of Pharmafocus!
Pharmafocus
pharmafocus.com
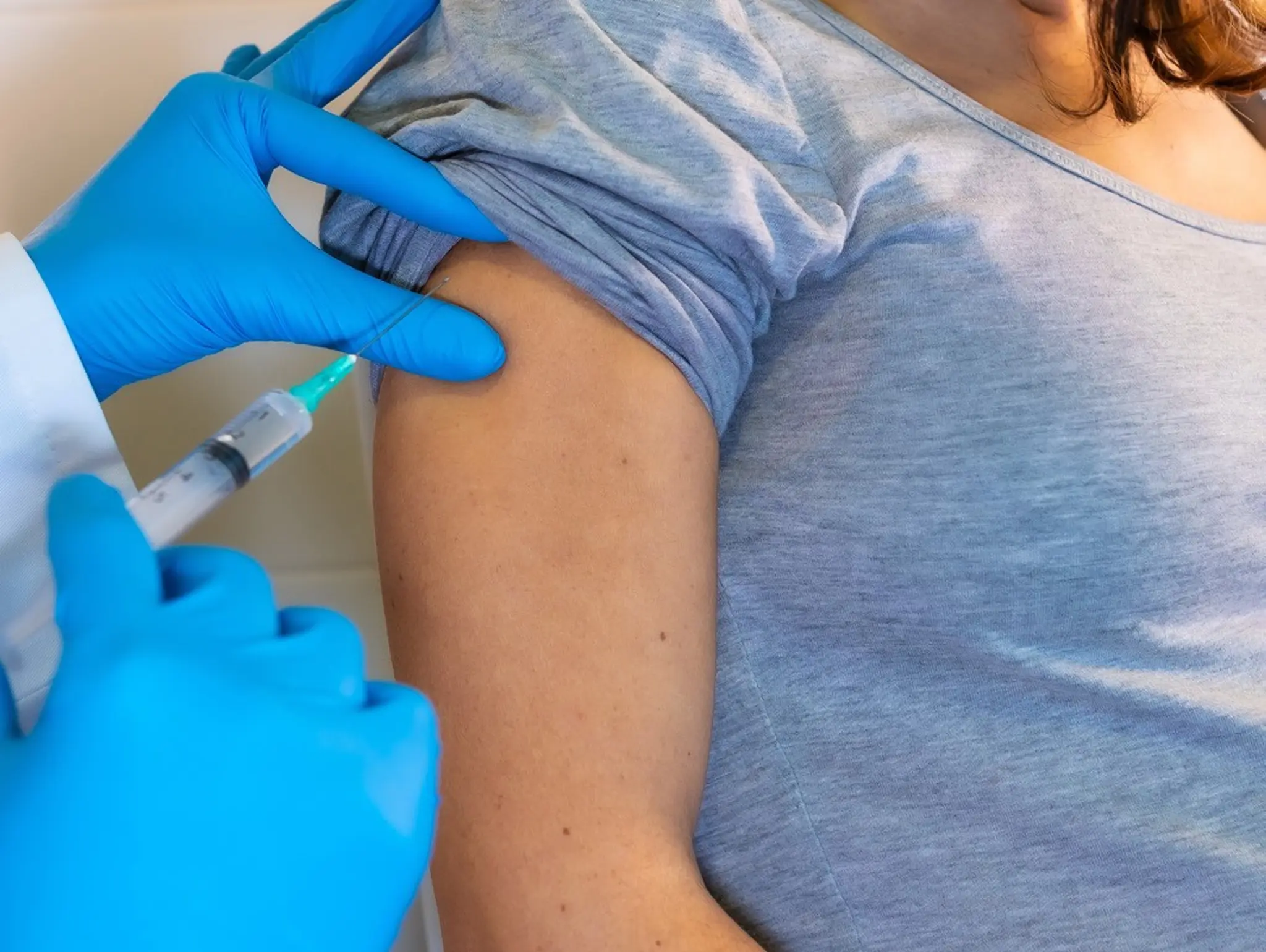
Pfizer’s maternal vaccine approved by FDA for prevention of RSV in infants
Pfizer has announced that the FDA has approved Abrysvo for the prevention of respiratory syncytial virus (RSV) in infants. The vaccine will work through the immunisation of pregnant women at 32 to 36 weeks of pregnancy.

Novo Nordisk’s Wegovy (semaglutide injection) made available in the UK
Novo Nordisk has today announced that Wegovy (semaglutide injection) is now available in the UK through a controlled and limited launch. The drug is intended to provide new treatment options to patients living with obesity.

Astellas plans opening of new facilities in Cambridge, Massachusetts, US
On 9 August 2023, Astellas Pharma announced its plans to open what it has described as a “state-of-the-art life sciences facility” in Divcowest’s Cambridge Crossing neighbourhood; the facility will be located at 441 Morgan avenue in Cambridge, Massachusetts, US.
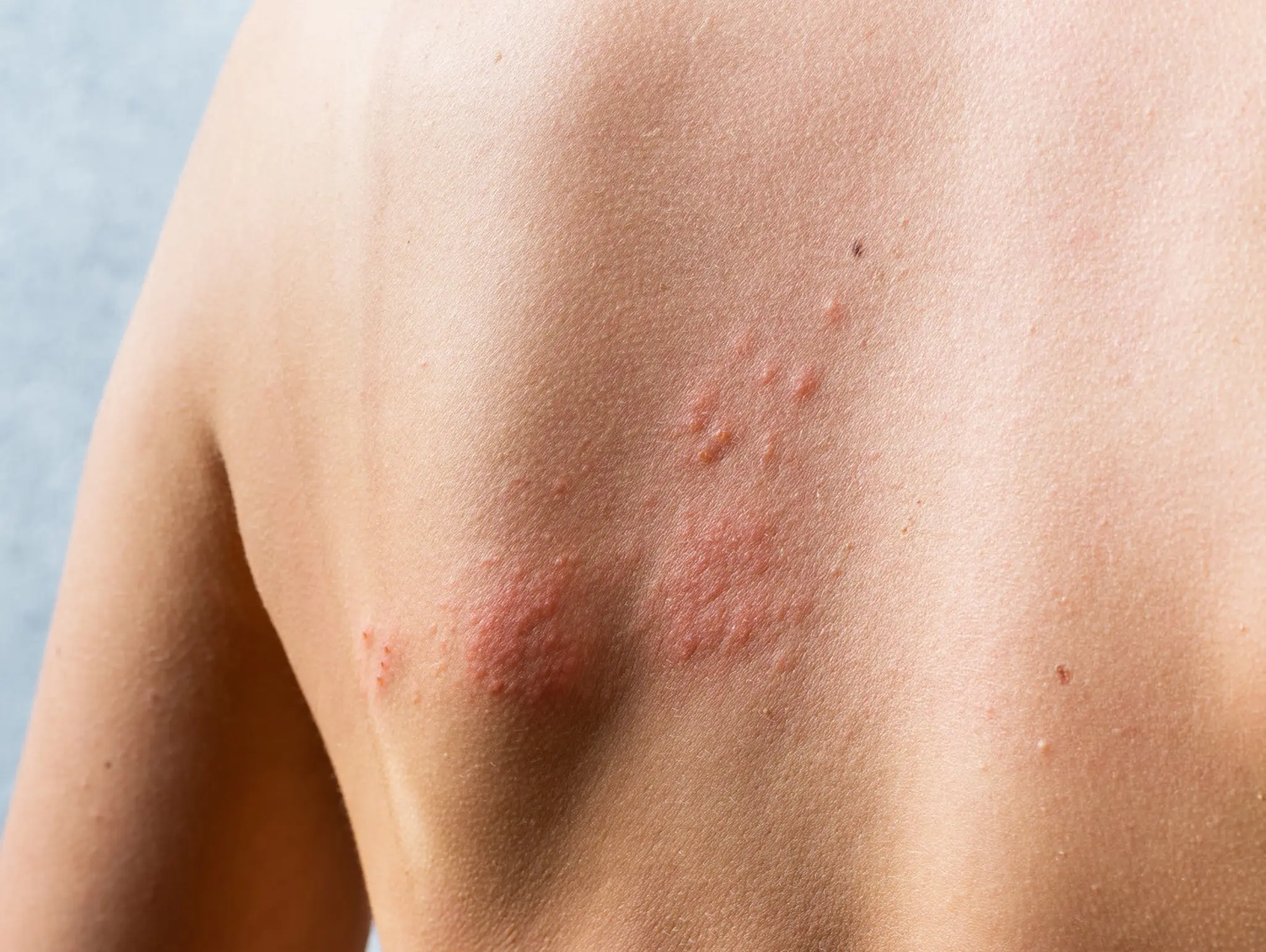
Promising results announced from GSK’s shingles trial in China
GSK has announced positive results from zoster-076 post-licence phase 4 efficacy trial for Shingrix recombinant zoster vaccine (RZV) in China.

FDA grants ODD to Faron Pharmaceuticals’ bexmarilimab for AML treatment
Faron Pharmaceuticals has announced that the FDA has granted orphan drug designation to bexmarilimab for the treatment of acute myeloid leukaemia (AML).

EMA accepts Iveric Bio’s MAA for avacincaptad pegol for treatment of GA secondary to AMD
Astellas Pharma has announced that the EMA has accepted the marketing authorisation application (MAA) for avacincaptad pegol (ACP) for the treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD).

Roche’s Genentech announces results from phase 3 ALK-positive early-stage lung cancer trial
Roche’s Genentech has announced that the phase 3 ALINA trial assessing Alecensa, compared with platinum-based chemotherapy for the treatment of early-stage anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC), met its primary endpoint of disease-free survival (DFS).

Eli Lilly’s Retevmo produces positive results in LIBRETTO-531 trial
Eli Lilly has announced topline results from the libretto-531 study assessing Retevmo (selpercatinib) as an initial treatment for patients with advanced or metastatic rearranged during transfection (RET)-mutant medullary thyroid cancer (MTC).
AstraZeneca & Daiichi Sankyo announce results from DESTINY-Lung02 trial of Enhertu for NSCLC
AstraZeneca and Daiichi Sankyo have announced results from the primary analysis of the destiny-lung02 phase 2 trial, which showed Enhertu’s (trastuzumab deruxtecan) continued strong and durable tumour responses in previously treated patients with her2-mutant (her2m) unresectable and/or metastatic non-squamous non-small cell lung cancer (NSCLC).

Merck’s phase 3 LITESPARK-005 trial of Welireg met primary endpoint
Merck has shared topline results from its phase 3 LITESPARK-005 trial, assessing Welireg (belzutifan) for the treatment of adult patients with advanced renal cell carcinoma (RCC) that has progressed following pd-1/l1 checkpoint inhibitor and vascular endothelial growth factor-tyrosine kinase inhibitor (vegf-tki) therapies.

Valneva and Pfizer’s Lyme disease vaccine candidate performs well in phase 2 trial
Valneva and Pfizer have announced positive paediatric and adolescent immunogenicity and safety data for their lyme disease vaccine candidate, vla15, when given as a booster.

CymaBay announces data from RESPONSE trial of seladelpar for primary biliary cholangitis treatment
Cymabay Therapeutics has announced positive topline results from its phase 3 RESPONSE study, which assessed the safety and efficacy of seladelpar for the treatment of adult patients with primary biliary cholangitis (PBC).

Pharma Role
Www.pharmarole.com

MHRA grants marketing authorisation to AbbVie’s Aquipta for migraine prevention
AbbVie has announced that the MHRA has granted a marketing authorisation for Aquipta (atogepant), for the prophylaxis of migraine in adult patients who have had at least four migraine days each month.
Roche’s Evrysdi approved by EC for babies under two months with spinal muscular atrophy
Roche has announced that the EC has approved the extension of Evrysdi’s marketing authorisation in the EU to include infants under two months with a clinical diagnosis of spinal muscular atrophy (SMA).

BMS’s Reblozyl gains FDA approval for anaemia treatment in adults with myelodysplastic syndromes
BMS has announced that the FDA has approved Reblozyl (luspatercept-aamt) for the treatment of anaemia without previous erythropoiesis stimulating agent use (esa-naïve) in adult patients with low- to intermediate-risk myelodysplastic syndromes (MDS) who may need regular red blood cell (RBC) transfusions.

Novartis’ Sandoz gains FDA approval for Tyruko biosimilar to treat MS
Novartis’ Sandoz has announced that the FDA has approved its biosimilar Tyruko (natalizumab-sztn), developed by Polpharma Biologics.

FDA approves new Vyvanse generics for ADHD and BED treatment amid ongoing shortages
The FDA has announced that it has approved several first generics of Takeda’s Vyvanse (lisdexamfetamine dimesylate) capsules and chewable tablets for attention-deficit/ hyperactivity disorder (ADHD) in patients over the age of six years and for moderate-to-severe binge-eating disorder (bed) in adult patients.
MHRA clears Amicus Therapeutics’ Pompe disease treatment for use in the UK
Amicus Therapeutics has announced that the MHRA in the UK has granted marketing authorisation to pombiliti (cipaglucosidase alfa) and opfolda (miglustat) for the treatment of adult patients with late-onset pompe disease.

Pharmacy app Charac receives £1.2m funding from NPA and pharmacy owners
Charac, an NHS-integrated pharmacy app, has raised £1.2m in debt and equity financing from the National Pharmacy Association (NPA) and pharmacy owners, bringing its total funds raised to £2.5m.

Sandoz shares exclusive deal to commercialise biosimilar ustekinumab
Sandoz has announced that it has entered into a development and commercialisation agreement with Samsung Bioepis, providing Sandoz the exclusive rights to commercialise the biosimilar sb17 ustekinumab in the US, Canada, EEA, Switzerland and the UK.
Tempus’ companion diagnostic test gains Breakthrough Device Designation from FDA
Tempus has announced that the FDA has granted it breakthrough device designation for its hla-loh assay as a companion diagnostic (cdx) test.
NICE publishes draft guidance surrounding the use of AI use in radiotherapy treatment planning
The National Institute for Health and Care Excellence (NICE) has shared draft guidance surrounding nine artificial intelligence (AI) technologies that can be used to speed up the planning of treatments for patients undergoing external beam radiotherapy for cancers including lung, prostate and colorectal.

Novo Nordisk acquires Embark Biotech
Embark Laboratories has announced that Novo Nordisk has officially acquired the biopharmaceutical company Embark Biotech, along with its lead asset metabolic programme.

Abbott shares plans to acquire Bigfoot Biomedical
Abbott and Bigfoot Biomedical have announced a definitive agreement for Abbott to acquire Bigfoot, however this transaction is still subject to customary closing conditions and is not expected to close until the third quarter of 2023.

Takeda & ImmunoGen announce collaboration for development and commercialisation of Elahere in Japan
ImmunoGen has announced that it has entered into an exclusive collaboration with Takeda Pharmaceutical Company for the development and commercialisation of Elahere (mirvetuximab soravtansine-gynx) in Japan.

FLYPHARMA EUROPE
Www.flypharmaeurope.com

How AI can advance today’s complex biopharma launch landscape
Florian Schnappauf from Veeva tells Pharmafocus about the role of generative AI in life sciences sales and marketing and how it can help bring new medicines to market faster
Unlocking science to discover novel solutions for breast cancer patients
Dr Martina Wiitzel from Daiichi Sankyo considers the burden of breast cancer highlighting the impact it has on women, and the advances in breast cancer care and precision medicines

Using genomics to uncover the causes of rare diseases and improve their treatment
Neil Ward from PacBio tells Pharmafocus about the use of whole genome sequencing in diagnosing and treating rare diseases, as well as considering other areas in which this could be utilised

Focusing on World Mental Health Day
Betsy Goodfellow from Pharmafocus looks at why mental health is so important during the colder, darker months

Innovations in Pharmaceutical Technology
Www.iptonline.com.website

Move of the month
Bristol Myers Squibb announces appointment of Dr Monica Shaw

Chris Tovey appointed as Destiny Pharma’s new CEO
Clinical stage biotech company Destiny Pharma has announced the appointment of Chris Tovey as chief executive officer.

Hansa Biopharma appoints Dr Hitto Kaufmann as chief scientific officer
Hansa Biopharma has announced the appointment of Dr Hitto Kaufmann as its new chief scientific officer effective 1 December 2023.

Dr Tomasz Kostrzewski introduced as CN Bio’s new chief scientific officer
CN Bio has announced the appointment of Dr Tomasz Kostrzewski as chief scientific officer

Five facts about mental health
Read on for five key facts about mental health.

Pharmafile
Www.pharmafile.com
