49 articles from this collection:
GSK’s RSV vaccine approved by MHRA
GSK has announced that the MHRA has authorised its Arexvy respiratory syncytial virus (RSV) vaccine for active immunisation for the prevention of lower respiratory tract disease (LRTD) caused by RSV in adults over the age of 60.

PMEA
Https://pmea.awardsplatform.com/

UKHSA opens new vaccine research centre in Wiltshire to prepare for future pandemics
Ministers have opened a new vaccine research centre at the UKHSA Porton Down campus in Wiltshire, intended to prepare for 'disease x', the next possible pandemic pathogen.

PharmaTimes COMMUNICATIONS AWARDS
Pharmatimes.com/communications_awards

Contents
Welcome to this issue of Pharmafocus!

Comment
Research innovations in rare diseases and improving patient access
Pharmafocus
Pharmafocus.com

Merck’s ebola vaccine, Ervebo, approved by FDA for children
On 3 August 2023, the FDA approved Merck’s Ervebo vaccine for children over 12 months.

Treatment programme for CVD to be developed as part of partnership between Novartis and Ionis
Ionis Pharmaceuticals has announced that it has entered into a new collaboration and licence agreement with Novartis, aiming to discover, develop and commercialise a novel medicine for patients with lipoprotein(a) or lp(a)-driven cardiovascular disease (CVD).

Contaminated cough syrup leads to WHO Medical Product Alert
The WHO has shared a medical product alert for an additional contaminated cough syrup medicine identified in Africa.

RNAi treatment for hypertension developed by Alnylam and Roche
Alnylam Pharmaceuticals has announced that it has entered a strategic agreement with Roche for the development and commercialisation of zilebesiran, Alnylam’s investigational RNAi therapeutic for the treatment of hypertension in patients with high cardiovascular risk.

GSK’s novel gonorrhoea vaccine gains Fast Track designation from FDA
GSK has announced that the FDA has granted fast track designation for its investigational vaccine for gonorrhoea, which is currently in an ongoing phase 2 trial assessing the efficacy of the vaccine in healthy adults aged 18 to 50 who are considered at risk of contracting gonorrhoea.

New open innovation hub opened by Astellas for tumour microenvironment research
Astellas Pharma and Mitsui Fudosan have announced that Astellas plans to establish a tumour microenvironment (TME) open innovation hub, intended to open in October 2023.

Data from Eli Lilly’s two phase 3 tirzepatide studies shared
Eli Lilly has announced results from its two phase 3 tirzepatide studies in adult patients with obesity or who are overweight with weight-related comorbidities, excluding type 2 diabetes.

First official long COVID-19 treatment could emerge from the NIH’s RECOVER initiative
As of 31 July 2023, the NIH launched the first trials of its ‘researching COVID to enhance recovery’ (RECOVER) initiative.

Study shows Wegovy by Novo Nordisk reduces cardiovascular risk
Novo Nordisk has shared results from the select cardiovascular outcomes trial, which show that a once-weekly subcutaneous dose of Wegovy (semaglutide 2.4mg) reduced the risk of major adverse cardiovascular events (mace) by 20% in overweight or obese adults over the age of 45 with established cardiovascular disease (CVD) and no prior history of diabetes.

Phase 2 trial data from GBS maternal vaccine candidate shared by Pfizer
Pfizer has announced new data from its phase 2 study, which assessed its hexavalent capsular polysaccharide conjugate group b streptococcus vaccine candidate, gbs6, in development for maternal vaccination in order to protect infants against invasive GBS disease.

Phase 3 trial results for datopotamab deruxtecan for NSCLC treatment announced by AstraZeneca
AstraZeneca has announced results from its tropion-lung01 phase 3 trial for its trop2-directed antibody drug conjugate, developed in collaboration with daiichi sankyo.

Breast cancer treatment phase 3 trial results shared by Merck
Merck, also known as MSD outside of the US and Canada, has announced positive results from the phase 3 KEYNOTE-756 trial, which assessed Keytruda in combination with chemotherapy for the treatment of patients with high-risk, early-stage oestrogen receptor-positive, human epidermal growth factor receptor 2-negative (er+/her2-) breast cancer.

PharmaRole
Pharmarole.com

Jemperli by GSK approved to treat endometrial cancer
GSK has announced that the FDA has approved Jemperli (dostarlimab) in combination with carboplatin and paclitaxel for the treatment of adult patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient (DMMR) or microsatellite instability-high (MSI-H).

Alzheimer’s disease drug Leqembi given full approval by FDA
Japanese Eisai and US-based biotechnology company Biogen have announced that the FDA has given their Alzheimer’s disease drug Leqembi (lecanemab-irmb) full approval through the approval of a supplemental biologics license application.

First over the counter contraceptive pill approved by FDA
FDA has announced that it has approved the Opill (norgestrel) tablet for non-prescription use to prevent pregnancy.

Bristol Myers Squibb’s Sotyktu recommended to treat adult patients with psoriasis on the NHS
Bristol Myers Squibb has announced that NICE has recommended Sotyktu (deucravacitinib) for use on the NHS in England as a new treatment option for adult patients with moderate-to-severe plaque psoriasis.

FDA approves first cellular therapy for treatment of patients with type 1 diabetes
The FDA has announced that it has approved Lantidra, the first allogeneic (donor) pancreatic islet cellular therapy made from deceased donor pancreatic cells for the treatment of adult patients with type 1 diabetes.

CHMP grants positive opinion to ViiV Healthcare’s cabotegravir for HIV prevention
GSK has announced that ViiV healthcare has received a positive opinion from the EMA's CHMP, recommending marketing authorisation for cabotegravir long-acting (LA) injectable and tablets for HIV prevention.

Data shared by Sanofi about new drug for treatment of haemophilia A in children
Sanofi has shared new data from the phase 3 xtend-kids study, which assessed Altuviiio (antihemophilic factor (recombinant), fc-vwf-xten fusion protein) once-weekly prophylaxis in patients under the age of 12 with previously treated severe haemophilia A.

American Heart Association grants $2.1m for research into link between CVD, migraines and strokes
The American Heart Association has announced that it will fund seven new scientific research studies to learn more about the link between migraines, strokes and cardiovascular disease (CVD).
New Crohn’s disease treatment could come from neonatal stem cells, research shows
Recent studies have discovered that human neonatal cardiac-derived mesenchymal stem cells can induce wound-healing and reduce inflammation in the digestive system, caused by Crohn’s disease.

Beclu carbon neutral asthma inhaler launched by Lupin Healthcare
Lupin Healthcare, has announced the UK launch of its carbon-neutral pressurised metered dose inhalers (PDMI) for the treatment of adult and paediatric patients with asthma.

AI-enabled drug discovery developments trigger partnership between Recursion and NVIDIA
Clinical stage biotech company Recursion has announced a $50m investment by NVIDIA, intended to accelerate the development of its AIfoundation models for biology and chemistry, working in collaboration with NVIDIA to optimise and distribute these ai models to biotechnology companies through NVIDIA's cloud services.

3D printed pills to enable time-controlled release in development
A team of scientists at the Max Planck Institute (MPI) for informatics, Germany, and the University of California, US, have developed a process of 3d printing pills in unusual shapes, allowing a time-controlled release as the object’s shape means it will dissolve in a predetermined manner.

Rare disease treatments to be researched in partnership between Pfizer and AstraZeneca’s Alexion
Alexion has announced that it has entered a definitive purchase and licence agreement with Pfizer.

Biogen plans to acquire Reata Pharmaceuticals for $7.3bn
Biogen and Reata Pharmaceuticals have announced that they have entered a definitive agreement under which Biogen will acquire Reata for approximately $7.3bn, or $172.50 per share.

Eli Lilly shares plans to acquire Versanis Bio for $1.9bn
Eli Lilly and Versanis Bio have announced a definitive agreement for Lilly to acquire Versanis, a private clinical-stage biopharmaceutical company focusing on the development of new medicines to treat cardiometabolic diseases.

FLYPHARMA EUROPE
Www.flypharmaeurope.com

Indication-based agreements – A key enabler of UK access to combination therapies in oncology
Jonathan Bowen from Sanofi UK and Ireland explains how indication-based agreements can remove barriers to patient access to combination cancer therapies
How statistical and data analysis can support research innovations in rare diseases
Giles Partington, Lindsay Govan, Paddy O’Hara, Emily Foreman and Jennifer Visser-Rogers from Phastar consider how data can be used to improve research innovations in rare diseases

PharmaTimes
Www.pharmatimes.com/intcr
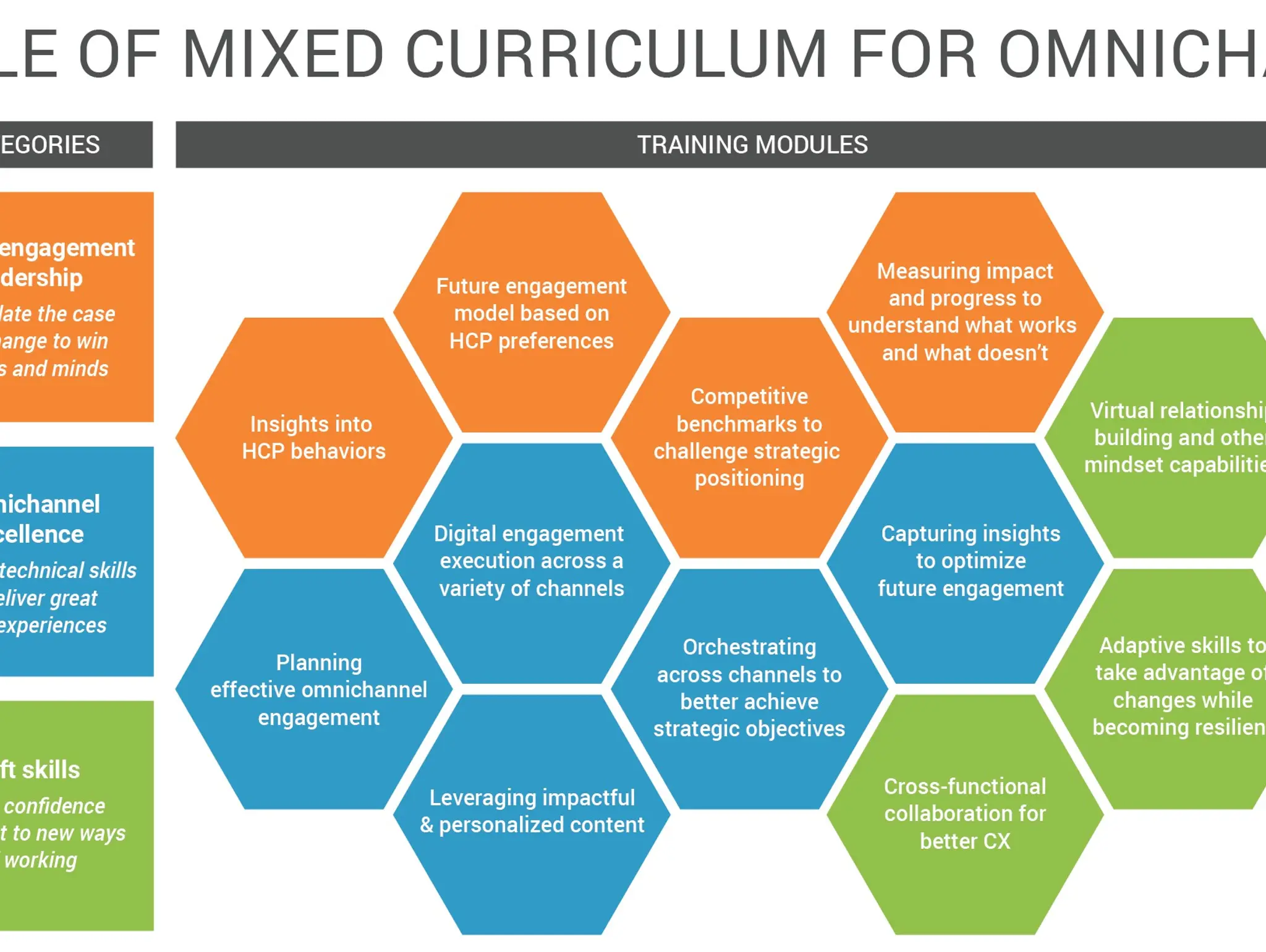
Seven ways to boost HCP engagement through field behavioural change
Veeva talks to Pharmafocus about the best ways that biopharma companies can boost their engagement

Why are so many community pharmacies closing and what does this mean for local communities?
Betsy Goodfellow from Pharmafocus considers the importance of community pharmacies as part of primary care in the UK and the significance of their closure

Innovations in Pharmaceutical Technology
Iptonline.com

Reacta appoints Kevin Hawkins as new head of regulatory affairs
Reacta appoints Kevin Hawkins as new head of regulatory affairs

Olav Hellebø to join Cytovation’s board of directors
Cytovation, a clinical stage immune-oncology company with a focus on targeted tumour membrane immunotherapies, has announced that it has appointed Olav Hellebø to its board of directors.

Helmholtz Centre for Infection Research appoints Josef Penninger to new scientific director
The Helmholtz Centre for Infection Research (HZI), Germany, has appointed professor Josef Penninger as scientific director.

Alfonso Quintás-Cardama to join Foghorn Therapeutics as new chief medical officer
The biotechnology company foghorn therapeutics has announced the addition of the new chief medical officer, Alfonso Quintás-Cardama, who will be replacing Sam Agresta after his retirement on 11 September 2023.

Five facts about rare diseases
Read on for five facts about rare diseases
Tweet us @ Pharmafocus
Follow us on twitter to keep up to date with breaking news. Tweet us and join in with our daily pharma polls! @Pharmafocus

Pharmafile
Www.pharmafile.com
