FDA grants approval to WISE for neuromonitoring cortical strip
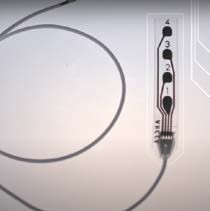
WISE, a medical equipment manufacturer based in Italy, has obtained FDA approval for its single-use medical device WISE Cortical Strip (WCS), used for Intraoperative Neurophysiological Monitoring (IONM). WCS is designed for intraoperative use with other medical equipment for recording, monitoring and stimulating the brain’s electrical signals.
Traditionally, cortical electrodes are made of stiff metal discs, with neuromonitoring and neuromodulation requiring surgical implantation of electrodes and leads into neural tissues to create stimuli or to measure electrical activity. However, WCS features stretchable platinum contacts embedded in a soft and thin film of silicone, allowing it to conform to the brain’s surface. Also, its unique Supersonic Technology enables the production of electrodes using stretchable electronic circuits integrated into thin elastomeric foils.
WISE has stated that its electrodes are highly ergonomic with superior adhesion while being minimally invasive and adaptable.
The FDA validated the performance of WCS in a multicentre clinical study, which showed the product to have superior performance with electrical impedance in physiological conditions, compared to conventional electrodes. It also had better adhesion, conformability and stability on the brain surface.
WISE CEO Luca Ravagnan said: “The FDA clearance is a crucial milestone for our commercial development, allowing us to expand the distribution of the WISE Cortical Strip from Europe to the US, and fuelling the development of the WISEneuro Monitoring product family. European clinicians are already demonstrating strong appreciation for the benefits of our product, we are looking forward to starting commercialisation also in the US.”